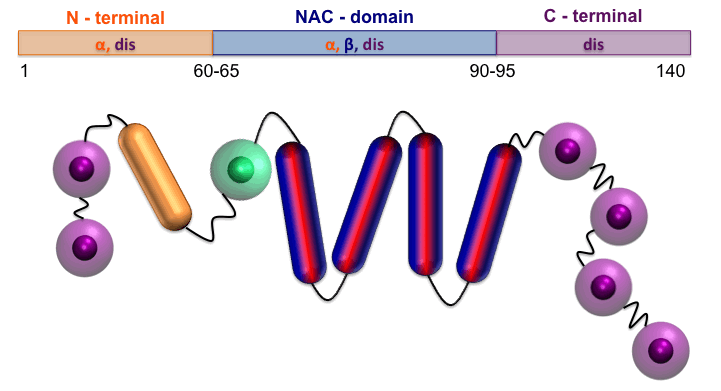
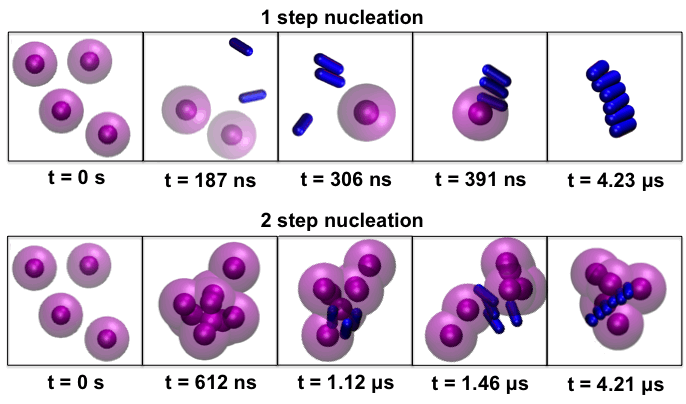
COMPUTATIONAL BIOPHYSICS
UNIVERSITY OF TWENTETHE NETHERLANDS
|
|
|
|
||
|
|
||||
|
|
|
welcome |
|
|
|
|
|
|
||
|
|
||||
|
|
||||
|
|
|
|
||
|
|
||||
|
|
|
people |
|
|
|
|
|
|
||
|
|
||||
|
|
||||
|
|
|
|
||
|
|
||||
|
|
|
highlights |
|
|
|
|
|
|
||
|
|
||||
|
|
||||
|
|
|
|
||
|
|
||||
|
|
|
research |
|
|
|
|
|
|
||
|
|
||||
|
|
||||
|
|
|
|
||
|
|
||||
|
|
|
publications |
|
|
|
|
|
|
||
|
|
||||
|
|
||||
|
|
|
|
||
|
|
||||
|
|
|
education |
|
|
|
|
|
|
||
|
|
||||
|
|
||||
|
|
|
|
||
|
|
||||
|
|
|
alumni |
|
|
|
|
|
|
||
|
|
||||
|
|
||||
|
|
|
|
||
|
|
||||
|
|
|
twentanglement |
|
|
|
|
|
|
||
|
|
||||
|
|
||||
|
|
|
|
||
|
|
||||
|
|
|
Briels trophy |
|
|
|
|
|
|
||
|
|
||||
|
|
||||
|
|
|
|
||
|
|
||||
|
|
|
downloads |
|
|
|
|
|
|
||
|
|
||||
|
|
||||
|
|
|
|
||
|
|
||||
|
|
|
vacancies |
|
|
|
|
|
|
||
|
|
||||
|
|
||||
|
|
|
|
||
|
|
||||
|
|
|
contact us |
|
|
|
|
|
|
||
|
|
||||
|
|
||||
|
|
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||